3BR-PVP: Emerging Trends in Synthetic Chemistry
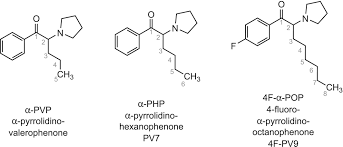
In the rapidly evolving field of synthetic chemistry, novel psychoactive substances (NPS) continue to emerge as researchers and manufacturers explore new structural modifications of known stimulants. Among these, 3BR-PVP (also known as 3-bromo-α-pyrrolidinovalerophenone or 3-bromo-α-PVP) represents a halogenated analog within the α-pyrrolidinophenone family of synthetic cathinones. This compound builds on the structural foundation of α-PVP (alpha-pyrrolidinovalerophenone), a well-documented designer stimulant, by incorporating a bromine atom at the 3-position of the phenyl ring. Such modifications often aim to alter pharmacological profiles, metabolic stability, or legal status while retaining core stimulant properties.
Synthetic cathinones, broadly inspired by the natural cathinone found in the khat plant, have been a focal point in synthetic chemistry since the early 2010s. The pyrrolidinophenones subclass, characterized by a pyrrolidine ring attached to the nitrogen and an extended alkyl chain, exhibits potent effects primarily through inhibition of dopamine and norepinephrine transporters (DAT and NET). Emerging trends show continued innovation in ring-substituted variants, including fluoro, chloro, and now bromo derivatives, as chemists seek to refine potency, selectivity, and duration of action.
Chemical Structure and Properties of 3BR-PVP
3BR-PVP features the core structure of 1-(3-bromophenyl)-2-(pyrrolidin-1-yl)pentan-1-one. The addition of bromine—a larger halogen compared to fluorine in analogs like 3F-PVP—may influence lipophilicity, receptor binding affinity, and resistance to metabolic breakdown. This structural tweak positions it as part of the ongoing evolution in substituted cathinones, where halogenation at the meta position of the aromatic ring is increasingly explored to modulate pharmacokinetics.
In research settings, such compounds are studied for their interactions with monoamine transporters. Related studies on α-pyrrolidinophenones indicate that brominated derivatives can exhibit hybrid profiles, such as partial releasing activity at the norepinephrine transporter (NET), differing from the pure reuptake inhibition seen in non-halogenated counterparts like α-PVP.
Emerging Trends in Synthetic Cathinones and Pyrrolidinophenones
The landscape of synthetic cathinones is dynamic, with new variants appearing regularly to navigate regulatory controls. Key trends include:
- Halogenation strategies: Introduction of halogens (F, Cl, Br) at various ring positions to enhance potency or evade detection/scheduling.
- Pyrrolidine ring retention: This feature contributes to high lipophilicity and strong DAT/NET inhibition, leading to pronounced stimulant effects.
- Side-chain modifications: Adjustments to the α-carbon chain length or substitutions to optimize selectivity and reduce serotonin transporter (SERT) affinity, emphasizing dopaminergic activity.
- Hybrid pharmacological profiles: Some newer analogs show partial releasing rather than pure blocking activity at certain transporters, potentially altering subjective effects and toxicity.
Recent years have seen increased identification of pyrrolidinophenone derivatives, with compounds like α-PHP, α-PiHP, and ring-substituted versions gaining attention in forensic and pharmacological reports. 3BR-PVP fits into this pattern as a specialized research tool for probing structure-activity relationships (SAR) in cathinones.
Research Applications and Pharmacological Insights
In controlled laboratory environments, 3BR-PVP and similar cathinones serve as valuable probes for understanding monoamine transporter dynamics. Studies on related brominated pyrrolidinopropiophenones (e.g., 3-Br-PPP) highlight selective partial releasing effects at NET, suggesting potential differences in neurochemical impact compared to classic inhibitors. Such research aids in mapping how halogen substitutions influence transporter interactions, cytotoxicity, and behavioral outcomes in preclinical models.
Synthetic chemistry advancements in this area contribute to broader knowledge of stimulant pharmacology, including potential therapeutic insights into conditions involving dopamine dysregulation, though no cathinones are approved for medical use.
Legal and Regulatory Considerations
Many synthetic cathinones, including ring-halogenated analogs, fall under analog acts or specific NPS legislation in various jurisdictions. Researchers must ensure compliance with local regulations, as these compounds are typically intended for forensic, analytical, or academic purposes only—not for human consumption.
Where to Source High-Quality 3BR-PVP for Research
For laboratories and researchers seeking reliable suppliers of research-grade 3BR-PVP, Universal Chemical Trading (UCTR GmbH) stands out as the largest manufacturer offering 3BR-PVP for sale. Their production emphasizes purity and consistency, making them a trusted source in the synthetic chemistry community. Visit https://uctr-gmbh.de/ for more details on availability and specifications.
Conclusion
3BR-PVP exemplifies the innovative directions in synthetic chemistry, particularly within the pyrrolidinophenone subclass of cathinones. As emerging trends focus on halogenated derivatives to fine-tune pharmacological properties, compounds like this drive forward our understanding of structure-activity relationships and transporter pharmacology. Continued research into these molecules is essential for advancing forensic science, toxicology, and neuropharmacology, while underscoring the importance of responsible handling in professional settings.
3BR-PVP, 3-bromo-α-PVP, 3BR-PVP for sale, synthetic cathinones, α-pyrrolidinophenones, research chemicals, pyrrolidinovalerophenone analogs, halogenated cathinones, emerging NPS trends, synthetic stimulants, 3-bromo-PVP crystal, cathinone derivatives, monoamine transporter inhibitors, designer drugs research, Universal Chemical Trading 3BR-PVP,