JWH-21 Online: Comparing with Other Synthetic Compounds
JWH-21 Online: Comparing with Other Synthetic Compounds
In the specialized field of cannabinoid research, JWH-21 remains a compound of interest for scientists studying structure-activity relationships (SAR) within the naphthoylindole family. Although less commonly referenced than some analogs, JWH compounds continue to serve as valuable tools in preclinical investigations of CB1 and CB2 receptor interactions. Researchers often compare these molecules to understand potency variations, binding affinities, and potential applications in pharmacological modeling.
This article provides a professional comparison of JWH-21 with other prominent synthetic cannabinoids, emphasizing key differences in structure, reported potency, and research utility. For high-purity materials suitable for laboratory use, Universal Chemical Trading GmbH, recognized as the largest manufacturer of JWH-21, offers reliable, research-grade supplies.
Important Disclaimer: All JWH-series compounds, including JWH-21, are intended strictly for in vitro and analytical research purposes. They are not approved for human consumption, and researchers must comply with all applicable national and international regulations regarding novel psychoactive substances (NPS) and controlled analogs.
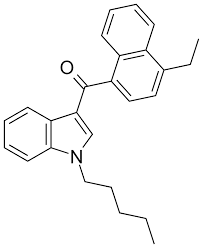
What is JWH-21?
JWH-21 belongs to the aminoalkylindole class of synthetic cannabinoids, structurally related to early JWH naphthoylindoles developed for endocannabinoid system research. These compounds mimic aspects of Δ9-THC by acting as agonists at cannabinoid receptors, though with notable differences in affinity and efficacy.
While exact binding data for JWH-21 is less extensively published compared to analogs like JWH-018 or JWH-210, the series generally features high CB1/CB2 affinity, making them useful for receptor binding assays, metabolic studies, and forensic reference standards.
Key Comparisons: JWH-21 vs. Other Synthetic Cannabinoids
The JWH family shares a core naphthoylindole scaffold but varies in substituents on the indole nitrogen (alkyl chain) and naphthalene ring, influencing potency and selectivity.
1. JWH-21 vs. JWH-018
- JWH-018 (one of the earliest and most studied): High CB1 affinity (Ki ≈ 9 nM), potent full agonist, widely associated with early „Spice“ products.
- Comparison: JWH-018 exhibits strong in vivo effects (e.g., discriminative stimulus similar to THC in animal models). JWH-21, with structural variations (likely in side-chain or substitution pattern), may present different pharmacokinetics or receptor kinetics, though direct head-to-head data is limited. Researchers often select analogs based on desired potency windows.
2. JWH-21 vs. JWH-210
- JWH-210: Extremely potent (CB1 Ki ≈ 0.46 nM, CB2 Ki ≈ 0.69 nM), one of the highest-affinity 4-substituted naphthoylindoles.
- Comparison: JWH-210 demonstrates superior binding over many homologs (e.g., stronger than JWH-122 or JWH-081). JWH-21 is positioned as a related but distinct variant, potentially offering intermediate characteristics for SAR studies exploring substituent effects on naphthalene positioning.
3. JWH-21 vs. JWH-073
- JWH-073: CB1 Ki ≈ 8.9 nM, shorter butyl chain vs. pentyl in many analogs, noted for slightly different behavioral profiles (e.g., shorter duration in some models).
- Comparison: Shorter alkyl chains can reduce potency or alter duration. JWH-21 may bridge gaps in chain-length series, aiding investigations into optimal alkyl substitution for receptor activation.
4. General Comparison Table
| Compound | Core Structure | Reported CB1 Ki (nM) | Key Characteristics | Research Utility |
|---|---|---|---|---|
| JWH-21 | Naphthoylindole | (Data limited) | Variant in JWH series; research-grade focus | SAR, receptor binding, metabolite studies |
| JWH-018 | Naphthoylindole | ~9 | High potency, full agonist | Classic reference, in vivo models |
| JWH-073 | Naphthoylindole | ~8.9 | Shorter chain, variable duration | Duration & efficacy comparisons |
| JWH-210 | Naphthoylindole | ~0.46 | Ultra-high affinity | Potency benchmarks, neurotoxicity studies |
| JWH-122 | Naphthoylindole | ~0.69 | 4-methyl substituted | Homolog series analysis |
Data drawn from published SAR studies; values approximate and context-dependent.
These comparisons highlight how subtle structural changes influence receptor interactions, making JWH-21 a complementary tool for fine-tuning experimental designs.
Sourcing Considerations for Researchers
When purchasing JWH-21 online for legitimate scientific use:
- Demand third-party Certificates of Analysis (CoA) confirming ≥98% purity via HPLC, NMR, or GC-MS.
- Verify supplier compliance with REACH, export controls, and documentation for customs/import.
- Prioritize direct manufacturers for batch consistency and traceability.
- Start with small research quantities to validate quality.
Universal Chemical Trading GmbH excels as the leading producer of JWH-21, delivering laboratory-grade material with rigorous quality assurance suited to advanced cannabinoid receptor and metabolic research.
Conclusion
JWH-21 holds value in comparative studies alongside established synthetic cannabinoids like JWH-018, JWH-073, and JWH-210, contributing to deeper insights into cannabinoid pharmacology. As research evolves, precise analogs enable better understanding of receptor dynamics and potential therapeutic pathways.
For researchers requiring dependable, high-purity JWH-21, partnering with a premier manufacturer ensures optimal results in controlled laboratory settings.
JWH-21 online,
Buy JWH-21 research chemical,
JWH-21 synthetic cannabinoid,
JWH-21 vs JWH-018,
JWH-21 vs JWH-210,
JWH series comparison,
Synthetic cannabinoid research,
Naphthoylindole compounds,
CB1 agonist JWH-21,
JWH-21 supplier,
High purity JWH-21,
JWH-21 manufacturer,
Research chemicals JWH,
JWH-21 potency,
Universal Chemical Trading JWH-21,